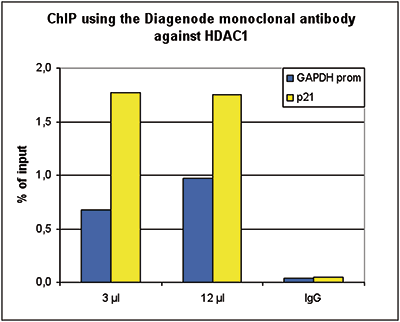
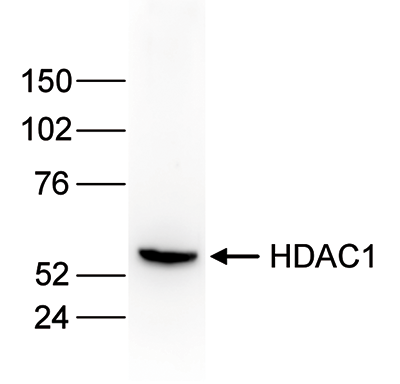
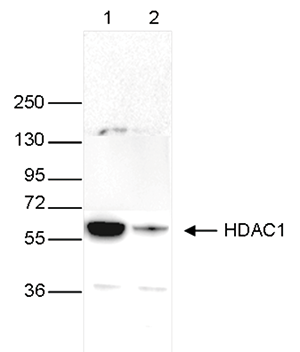
HDAC1 (UniProt/Swiss-Prot entry Q13547) catalyses the deacetylation of lysine residues on the N-terminal part of the core histones (H2A, H2B, H3 and H4). Acetylation and deacetylation of these highly conserved lysine residues is important for the control of gene expression and HDAC activity is associated with gene repression. Histone deacetylation is established by the formation of large multiprotein complexes. HDAC1 also interacts with the retinoblastoma tumor suppressor protein and is able to deacetylate p53. Therefore, it plays an essential role in cell proliferation and differentiation and in apoptosis.