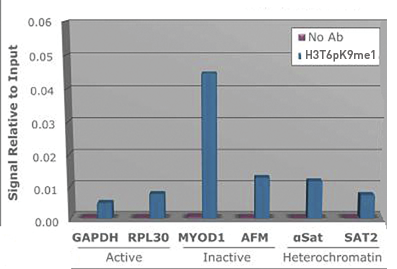
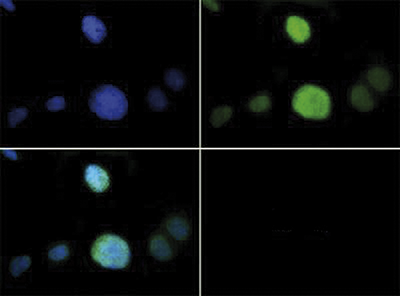
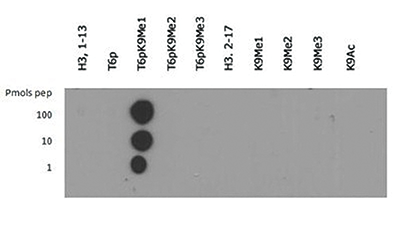
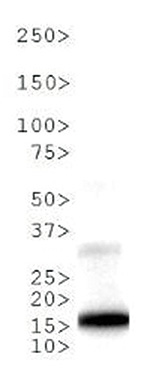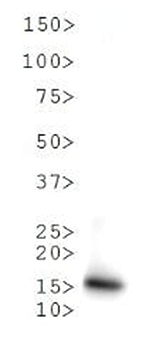Methylation of Histone H3 at Lys9 (K9) is an epigenetic silencer of transcription. Gene silencing from histone post translational modifications, as well as DNA methylation, play a key role in the development of normal tissues. If this silencing is disturbed through the artificial silencing of RIZ1, and thereby H3 K9Me1, it has been shown that normal apoptotic processes in precancerous cells can be reduced. Interestingly, data indicates that the conversion of the monomethyl to the trimethyl form requires mediation by SUVR4 in transposons and pseudogenes. Research also indicates that the presence of the G9a/GLP heterodimeric complex is required for this modification to exist. The additional phosphorylation at Thr6 (T6p) affects the ability of other proteins to bind to the H3 tail, along with amplifing the effects of other histone PTMs that are present. Because T6 phosphorylation is constitutive, its dephosphorylation may play a key role in DNA transcription, repair and replication.