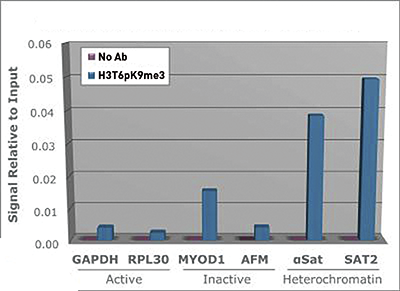
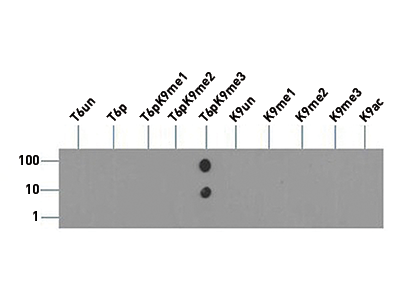
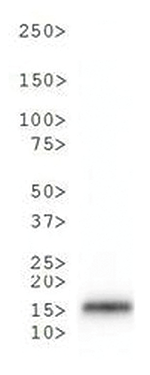Phosphorylation as a post-translational modification on histone H3 modulates the ability of proteins to recognize and bind to the H3 tail. When additional modifications to H3 are present, in addition to the native T6 phosphorylation, there is an amplified effect. Several authors suggest that the complexity of these combined PTMs on histone tails should be described as a convoluted language, rather than as a strict code. Since the H3T6 phosphorylation seems to be constitutively present, its de-phosphorylation could be highly significant and affect transcription, repair and replication.