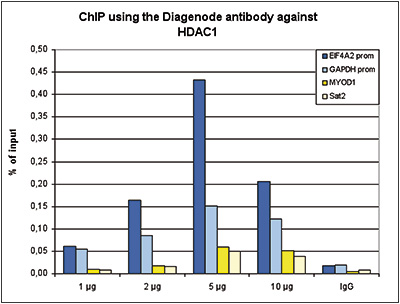
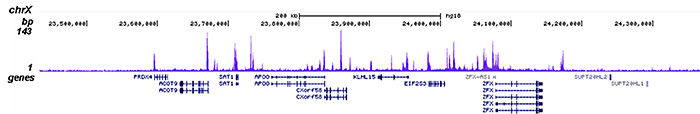
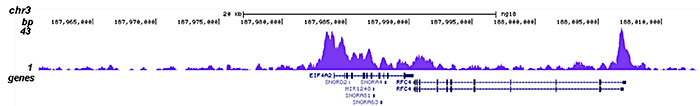
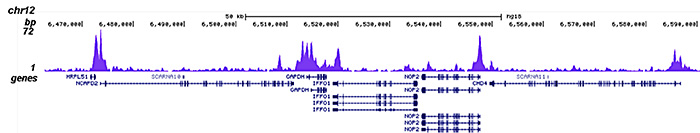
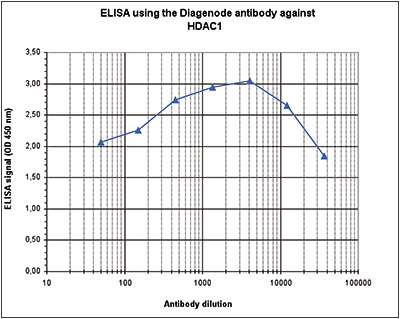
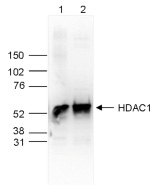
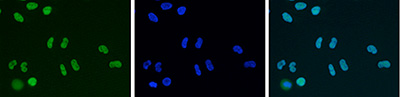
How to properly cite our product/service in your work We strongly recommend using this: HDAC1 Antibody - ChIP-seq Grade - replaced by the reference C15410325 (Hologic Diagenode Cat# C15410053 Lot# A21-001P). Click here to copy to clipboard. Using our products or services in your publication? Let us know! |
Comprehensive molecular phenotyping of -deficient gastric cancer revealspervasive epigenomic reprogramming and therapeutic opportunities.
Xu C. et al.
OBJECTIVE: Gastric cancer (GC) is a leading cause of cancer mortality, with being the second most frequently mutated driver gene in GC. We sought to decipher -specific GC regulatory networks and examine therapeutic vulnerabilities arising from loss. DESIGN: Genomic profiling of GC patients including a Singapore coho... |
Functional incompatibility between the generic NF-κB motif and a subtype-specific Sp1III element drives the formation of HIV-1 subtype C viral promoter
Verma A et al.
Of the various genetic subtypes of HIV-1, HIV-2 and SIV, only in subtype C of HIV-1, a genetically variant NF-κB binding site is found at the core of the viral promoter in association with a subtype-specific Sp1III motif. How the subtype-associated variations in the core transcription factor binding sites (TFB... |
Genome-wide hydroxymethylcytosine pattern changes in response to oxidative stress
Delatte B, Jeschke J, Defrance M, Bachman M, Creppe C, Calonne E, Bizet M, Deplus R, Marroquí L, Libin M, Ravichandran M, Mascart F, Eizirik DL, Murrell A, Jurkowski TP, Fuks F
The TET enzymes convert methylcytosine to the newly discovered base hydroxymethylcytosine. While recent reports suggest that TETs may play a role in response to oxidative stress, this role remains uncertain, and results lack in vivo models. Here we show a global decrease of hydroxymethylcytosine in cells treated w... |
SNAIL1 combines competitive displacement of ASCL2 and epigenetic mechanisms to rapidly silence the EPHB3 tumor suppressor in colorectal cancer.
Rönsch K, Jägle S, Rose K, Seidl M, Baumgartner F, Freihen V, Yousaf A, Metzger E, Lassmann S, Schüle R, Zeiser R, Michoel T, Hecht A
EPHB3 is a critical cellular guidance factor in the intestinal epithelium and an important tumor suppressor in colorectal cancer (CRC) whose expression is frequently lost at the adenoma-carcinoma transition when tumor cells become invasive. The molecular mechanisms underlying EPHB3 silencing are incompletely underst... |
Citrullination of DNMT3A by PADI4 regulates its stability and controls DNA methylation.
Deplus R, Denis H, Putmans P, Calonne E, Fourrez M, Yamamoto K, Suzuki A, Fuks F
DNA methylation is a central epigenetic modification in mammals, with essential roles in development and disease. De novo DNA methyltransferases establish DNA methylation patterns in specific regions within the genome by mechanisms that remain poorly understood. Here we show that protein citrullination by peptidylar... |
Dimethyl fumarate regulates histone deacetylase expression in astrocytes.
Kalinin S, Polak PE, Lin SX, Braun D, Guizzetti M, Zhang X, Rubinstein I, Feinstein DL
We previously showed that dimethyl fumarate (DMF) reduces inflammatory activation in astrocytes, involving activation of transcription factor Nrf2. However, the pathways causing Nrf2 activation were not examined. We now show that DMF modifies expression of histone deacetylases (HDACs) in primary rat astrocytes. Afte... |
Phosphorylation of p65(RelA) on Ser547 by ATM Represses NF-κB-Dependent Transcription of Specific Genes after Genotoxic Stress
Sabatel H, Di Valentin E, Gloire G, Dequiedt F, Piette J, Habraken Y
|
The histone demethylase Kdm3a is essential to progression through differentiation.
Herzog M, Josseaux E, Dedeurwaerder S, Calonne E, Volkmar M, Fuks F
Histone demethylation has important roles in regulating gene expression and forms part of the epigenetic memory system that regulates cell fate and identity by still poorly understood mechanisms. Here, we examined the role of histone demethylase Kdm3a during cell differentiation, showing that Kdm3a is essential for ... |
HDAC1 Regulates Fear Extinction in Mice.
Bahari-Javan S, Maddalena A, Kerimoglu C, Wittnam J, Held T, Bähr M, Burkhardt S, Delalle I, Kügler S, Fischer A, Sananbenesi F
Histone acetylation has been implicated with the pathogenesis of neuropsychiatric disorders and targeting histone deacetylases (HDACs) using HDAC inhibitors was shown to be neuroprotective and to initiate neuroregenerative processes. However, little is known about the role of individual HDAC proteins during the path... |
Enhancer of Zeste 2 (EZH2) is up-regulated in malignant gliomas and in glioma stem-like cells.
Orzan F, Pellegatta S, Poliani PL, Pisati F, Caldera V, Menghi F, Kapetis D, Marras C, Schiffer D, Finocchiaro G
AIMS: Proteins of the Polycomb repressive complex 2 (PRC2) are epigenetic gene silencers and are involved in tumour development. Their oncogenic function might be associated with their role in stem cell maintenance. The histone methyltransferase Enhancer of Zeste 2 (EZH2) is a key member of PRC2 function: we have in... |
The core binding factor CBF negatively regulates skeletal muscle terminal differentiation.
Philipot O, Joliot V, Ait-Mohamed O, Pellentz C, Robin P, Fritsch L, Ait-Si-Ali S
BACKGROUND: Core Binding Factor or CBF is a transcription factor composed of two subunits, Runx1/AML-1 and CBF beta or CBFbeta. CBF was originally described as a regulator of hematopoiesis. METHODOLOGY/PRINCIPAL FINDINGS: Here we show that CBF is involved in the control of skeletal muscle terminal differentiation. I... |
Functional connection between deimination and deacetylation of histones.
Denis H, Deplus R, Putmans P, Yamada M, Métivier R, Fuks F
Histone methylation plays key roles in regulating chromatin structure and function. The recent identification of enzymes that antagonize or remove histone methylation offers new opportunities to appreciate histone methylation plasticity in the regulation of epigenetic pathways. Peptidylarginine deiminase 4 (PADI4; a... |