The NGS Competence Center Tübingen (NCCT), together with four other national centers, has been established by the DFG (German Research Foundation) to support research projects with diverse needs of high-throughput sequencing technologies.
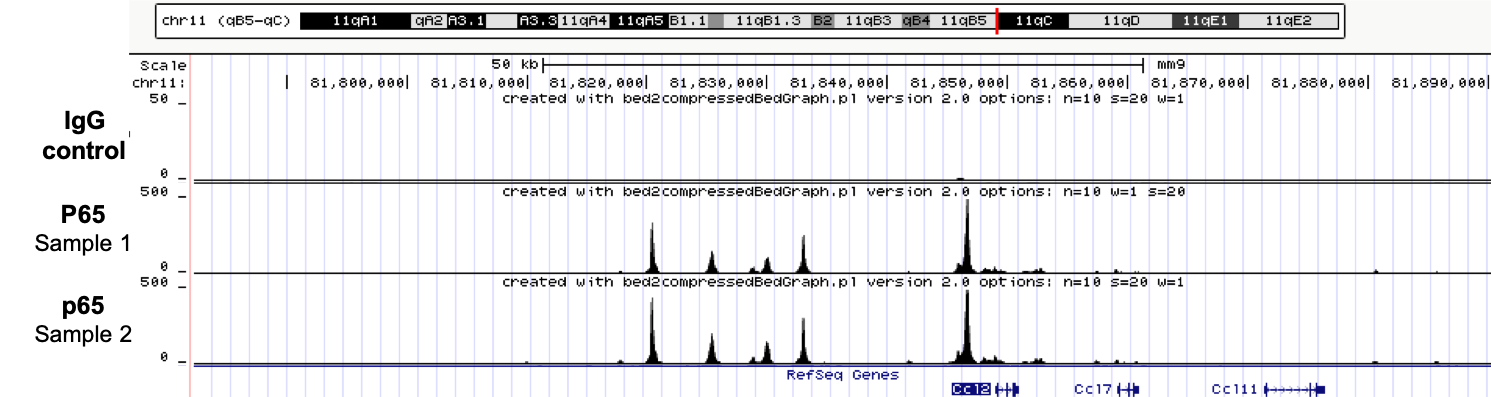
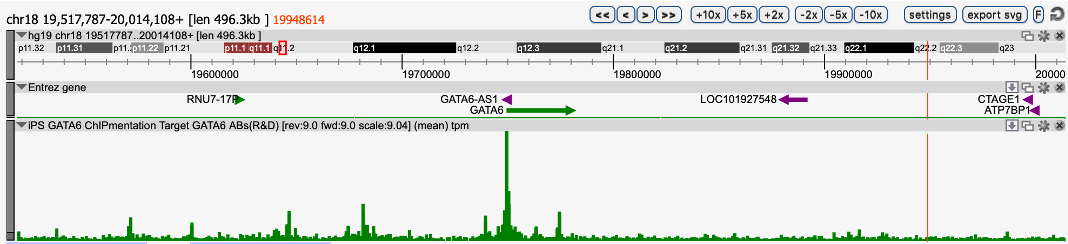
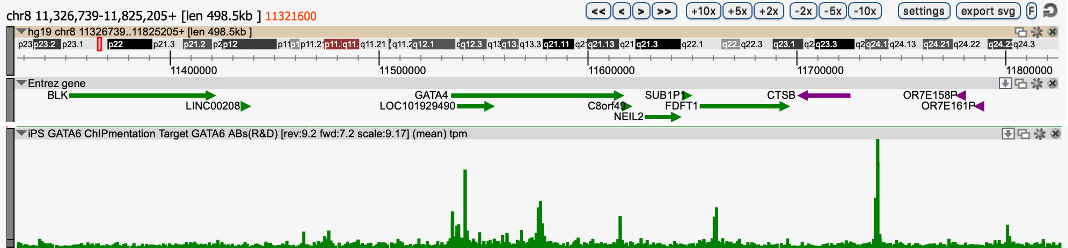
Long-read sequencing is very helpful to answer scientific questions in various topics such as microbiology or clinical research. We have noticed that the data yield of Nanopore sequencing can be notably increased by shearing the high molecular weight genomic DNA with an average size distribution of ~30kb and obtaining a read length N50 of 30kb. In this context, the Megaruptor 3 was critical to achieve long, homogenous and reproducible DNA preparation.
Megaruptor 3 is able to shear different molecular weight ranges up to 100kb; provided the input genomic DNA is of high-molecular weight. We have tested the Megaruptor 3 with genomic DNA from human blood, fibroblasts and difficult samples such as bacterial genomic DNA with high viscosity. With the Megaruptor 3 we have easily sheared up to 8 samples in parallel, saving preparation time. We have tested concentrations as low as 5 ng/µL and up to 70 ng/µL, saving sample material for optimization and meeting downstream requirements for library preparation.
Finally, handling of the Megaruptor 3 is quick, with a simple interface. Diagenode is fast in delivering consumables and these are ready-to-go. Sample preparation requires one pipetting step. You need to enter 2 parameters of your sample: volume and concentration in addition to the speed related to your desired size. It is a safe process without sample cross-contamination. It is easy to control whether the sample is going through the hydropore. The system is fast; for the concentration and speed conditions we have tested, runs were completed between half an hour and 2 hours.
Elena Buena Atienza and Dr. Nicolas Casadei, Institute of Medical Genetics and Applied Genomics, University Clinics Tübingen
about Megaruptor® 3.